Microsoft word - epiduo pcb final feb 2010.doc
London New Drugs Group February 2010 RIMARY CARE BRIEFING: Epiduo 0.1%/2.5% gel This Primary Care Briefing has been produced to inform healthcare professionals in primary care of the new Epiduo, which is used for mild to moderate acne when comedones, papules and pustules are present. What is Epiduo? What is Epiduo licensed for? Epiduo is a topical gel which contains adapa
 Separation Science and Technology, 40: 1555–1566, 2005Copyright # Taylor & Francis, Inc.
Separation Science and Technology, 40: 1555–1566, 2005Copyright # Taylor & Francis, Inc.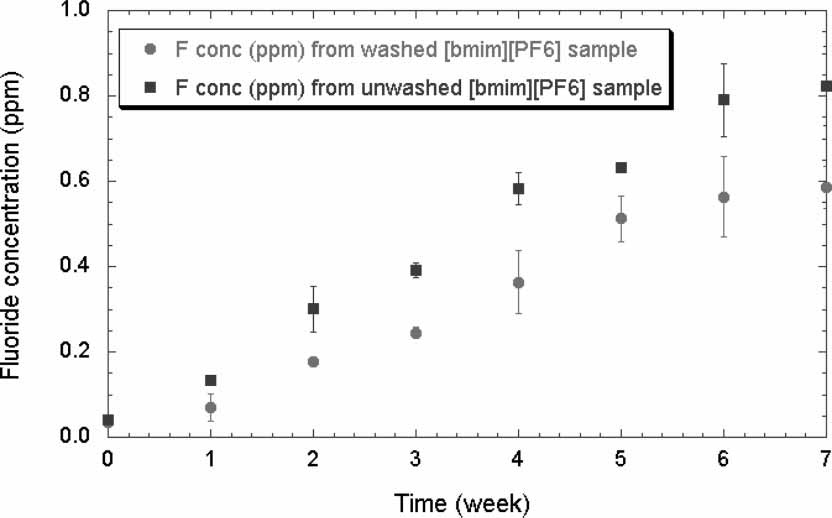 Partitioning Behavior of Tyramine and 2-Methoxyphenethylamine
Fluoride concentration in water, which had been continuously shaken with
washed and unwashed [bmim][PF6] samples over time.
Partitioning Behavior of Tyramine and 2-Methoxyphenethylamine
Fluoride concentration in water, which had been continuously shaken with
washed and unwashed [bmim][PF6] samples over time.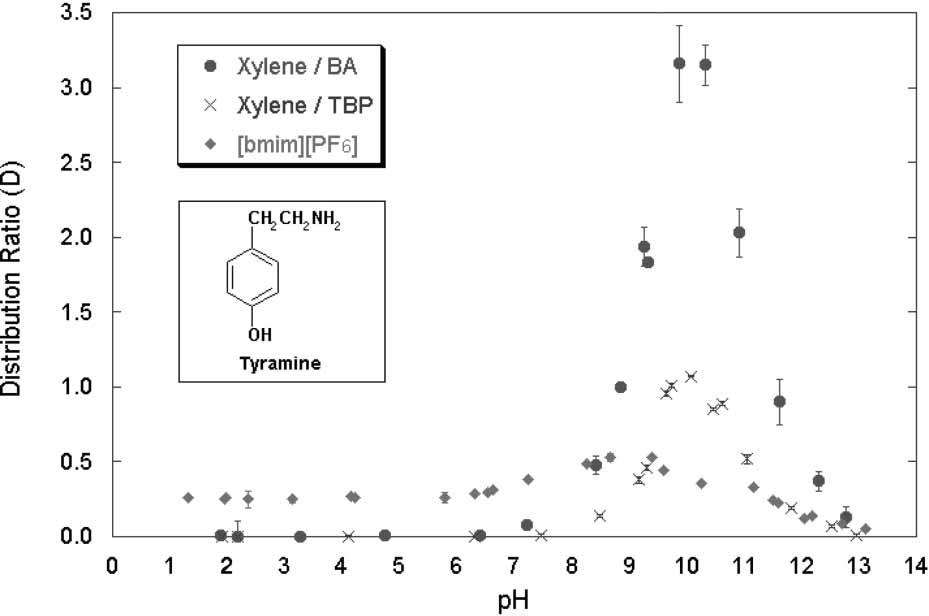 Distribution ratio vs. pH with tyramine as solute in [bmim][PF6]/water,
xylene/TBP/water and xylene/BA/water systems.
Distribution ratio vs. pH with tyramine as solute in [bmim][PF6]/water,
xylene/TBP/water and xylene/BA/water systems.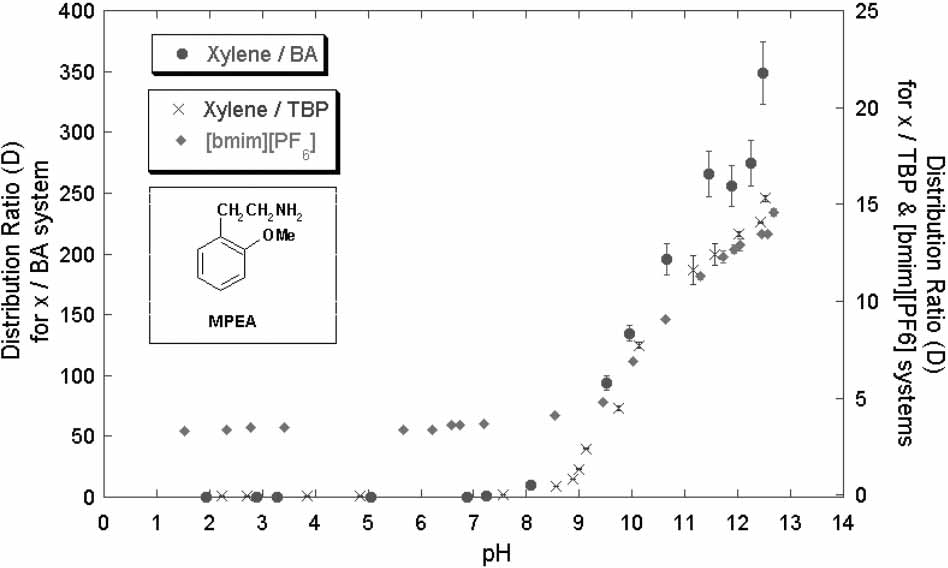 Partitioning Behavior of Tyramine and 2-Methoxyphenethylamine
Distribution ratio vs. pH with MPEA as solute in [bmim][PF6]/water,
xylene/TBP/water and xylene/BA/water systems.
Partitioning Behavior of Tyramine and 2-Methoxyphenethylamine
Distribution ratio vs. pH with MPEA as solute in [bmim][PF6]/water,
xylene/TBP/water and xylene/BA/water systems.