Microsoft word - dermedicaconsultcard.docx
Name _________________________________________________________________________________________________ Address _______________________________________________________________________________________________ _________________________________________________________________________________________________ Phone ______________________________________________________ email: ________________________
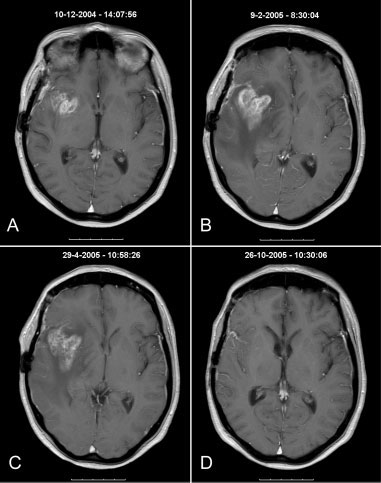 progression. In only 1 patient could the decreaseddose of dexamethasone explain the observed pro-gression noted on the MRI scan (Patient 17). Threepatients did not continue with adjuvant TMZbecause of neurologic deterioration (Patients 22, 31,and 34). All of the remaining patients continued withadjuvant TMZ.
progression. In only 1 patient could the decreaseddose of dexamethasone explain the observed pro-gression noted on the MRI scan (Patient 17). Threepatients did not continue with adjuvant TMZbecause of neurologic deterioration (Patients 22, 31,and 34). All of the remaining patients continued withadjuvant TMZ. Pseudo-progression in Glioma Patients/Taal et al.
Pseudo-progression in Glioma Patients/Taal et al.